New catalyst could provide liquid hydrogen fuel of the future
Researchers at Lund University are investigating a car fuel comprised of a liquid that is converted to hydrogen by a solid catalyst. The used liquid is then emptied from the tank and charged with hydrogen, after which it can be used again in a circular system that is free from greenhouse gas emissions.
Jonas Andersson – Published 13 October 2023
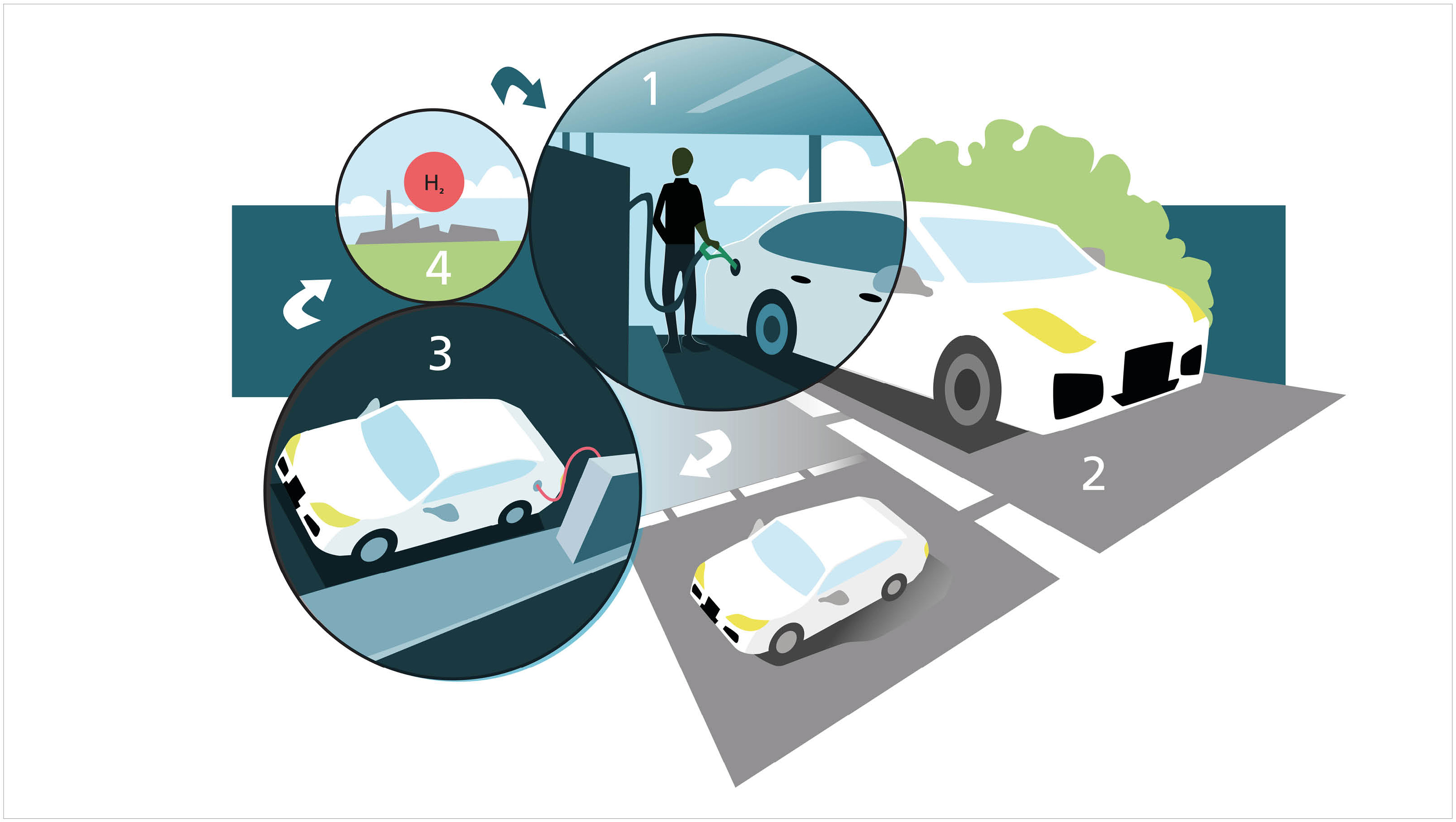
In two research articles, Lund researchers have demonstrated that the method works, and while it is still basic research, it has the potential to become an efficient energy-storage system in the future.
“Our catalyst is one of the most efficient around, at least if you look at publicly available research,” says Ola Wendt, professor at the Department of Chemistry at Lund University, and one of the authors.
Liquid fuel charged with hydrogen
Finding alternative ways of producing, storing and transforming energy in order to reduce carbon dioxide emissions from fossil fuels is necessary to reduce the impact on the climate. One way involves much-talked-about hydrogen gas, which many see as a future solution for energy storage. Nature stores energy in chemical bonds, and hydrogen contains the highest energy density in relation to its weight.
“However, gas can be difficult to handle, so we are looking at liquid fuel charged with hydrogen that can be delivered at a pump, in a way broadly similar to what happens at petrol stations today,” says Ola Wendt.
The concept is known as LOHC (liquid organic hydrogen carriers) and is not new as such. The challenge is in finding as efficient a catalyst as possible, that can extract the hydrogen from the liquid.
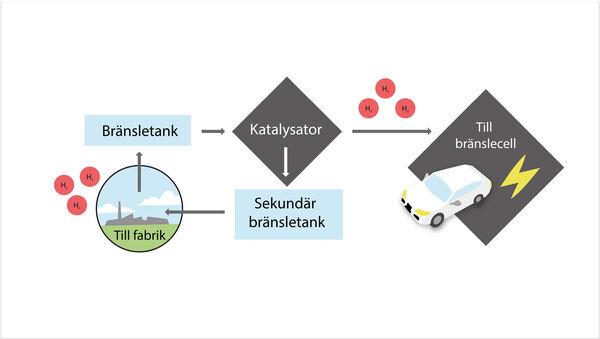
The tank is a circular system where hydrogen is extracted from the liquid via a catalyst. The used liquid is emptied and recharged with hydrogen. Illustration: Catrin Jakobsson
More than 99 per cent converation
The system is intended to work using a liquid that is “charged” with hydrogen. The liquid is pumped through a solid catalyst which extracts the hydrogen. This can be used in a fuel cell – which converts chemical fuel to electricity – while the “spent” liquid carries on to another tank. The only emission is water.
The spent liquid can then be emptied at a filling station before refuelling with new, charged liquid. This would probably mean large scale production of the substance, comparable to today’s oil refineries.
“We converted more than 99 per cent of the hydrogen gas that was present in the liquid,” says Ola Wendt.
Possible for larger vehicles
Researchers have also been calculating whether it might be possible to use the fuel for larger vehicles such as buses, trucks and aircraft.
“With the large tanks that they have, it might be possible to cover almost the same distance as you can with a tank of diesel. You would also convert around 50 per cent more energy compared to compressed hydrogen,” says Ola Wendt.
The liquids used are isopropanol (which is a common ingredient in screenwash) and 4-methylpiperidine.
Does this sound a little too good to be true? Yes – for now at least, a number of challenges remain. One is that the lifespan of the catalyst is rather limited. Another is that iridium, which the catalyst is based on, is a precious metal.
“But we estimate that you need about two grams of iridium per car. This could be compared to today’s exhaust-cleaning catalytic converters, which contain about three grams of platinum, palladium and rhodium, which are also precious metals,” says Ola Wendt.
This is a technical solution based on basic research. If a decision was made to go for a finished product, Ola Wendt believes that the concept could be ready in ten years’ time – provided that it is economically viable and that there is interest from society.
Political descisions requiered
Another problem is how hydrogen is produced – today, most production is not climate friendly. The hydrogen then needs to be stored and transported in an effective way, which is not that straightforward today. There are also the risks of refuelling with compressed hydrogen. The Lund researchers hope to solve this with their method.
“Ninety-eight per cent of all hydrogen today is fossil-based, produced from natural gas. The biproduct is carbon dioxide. From an environmental point of view, the notion of producing hydrogen for steel, batteries and fuel is pointless if it is done using natural gas,” says Ola Wendt, but he explains that there is a lot of research going on into how “green hydrogen” might be produced by splitting water into hydrogen and oxygen with the help of renewable energy.
At the same time, Ola Wendt believes that political decisions are required for renewable and climate-friendly alternatives to gain a proper foothold.
“It needs to be cheaper, and it takes political decisions. Renewables have no chance of competing with something that you just dig out of the ground, where transport is almost the only cost, as is the case with fossil fuels,” he concludes.
Publications:
Hydrogen and energy
Hydrogen gas has a high energy density in relation to weight, 33kWh/kg (by comparison: 13 kWh/kg for petrol and <0.25 kWh/kg for lithium-ion batteries – the reason electric cars have a limited range).
It is however difficult to store hydrogen gas since the substance is a gas in atmospheric conditions and therefore has an extremely low volume density. To retain the important advantages of liquid fuels such as petrol and diesel, i.e. relative safety, quick refuelling, high energy density and compatibility with existing infrastructure, it has been suggested that hydrogen be stored in liquid material. These liquid organic hydrogen carriers (LOHC) must be passed through a catalytic reactor, where hydrogen is released and led to a fuel cell.

