From pulse to picture
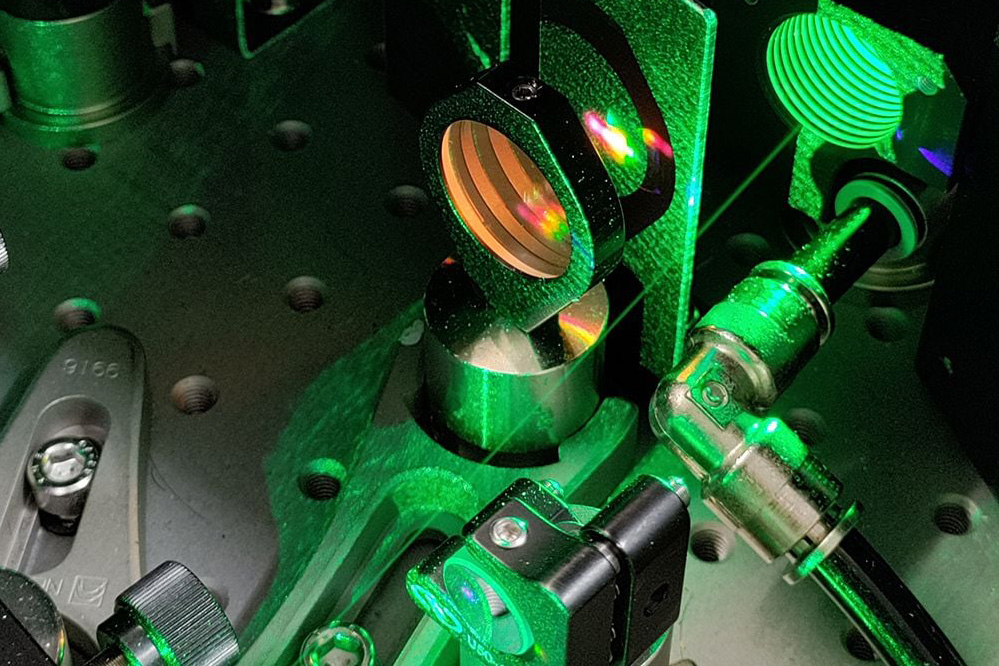
First, the researchers switch on a laser with infrared light pulses. These pulses are on the femtosecond scale, lasting a millionth of a billionth of a second.
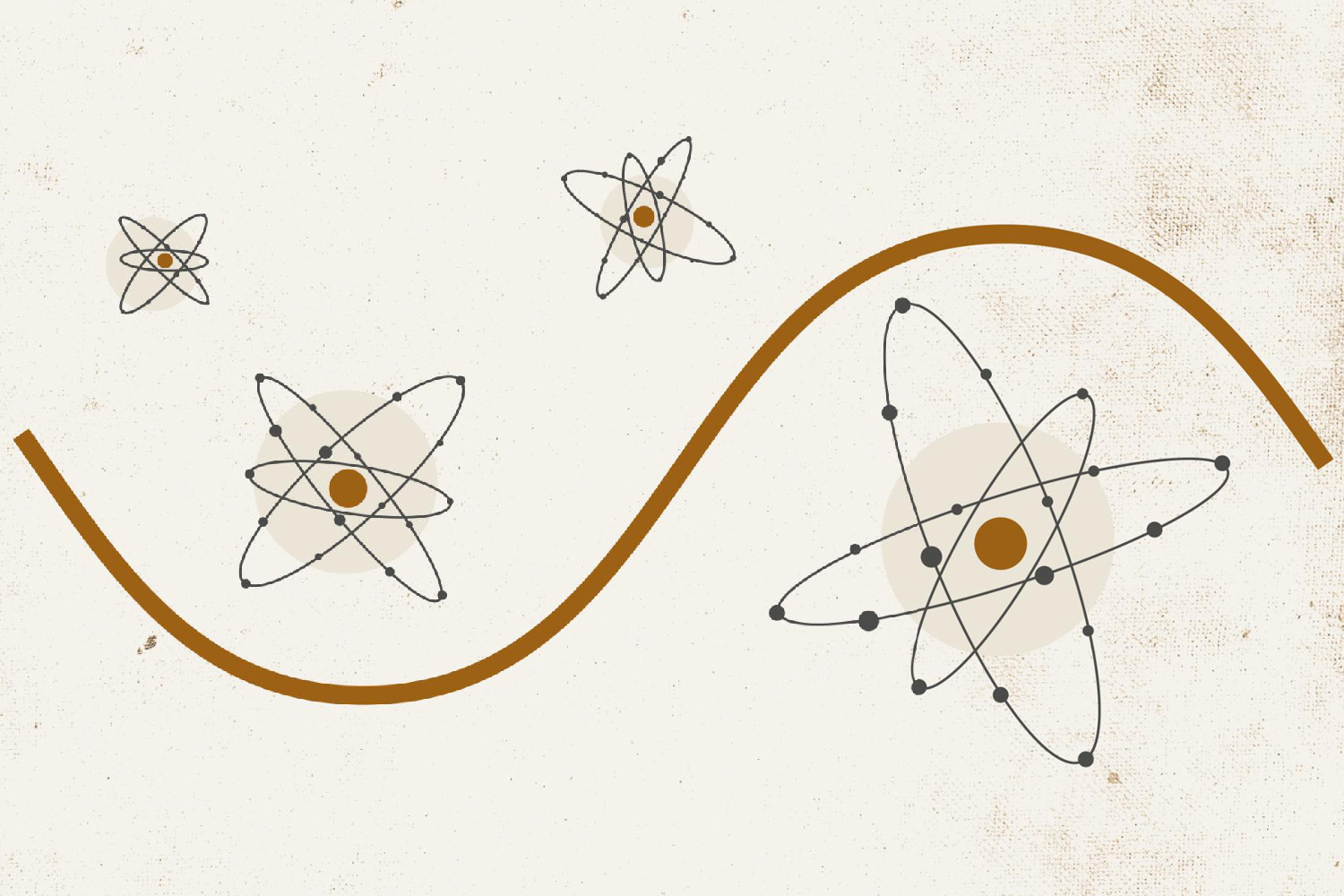
A concentrated laser beam is directed at a noble gas. Why a noble gas? Gases allow the irradiation of all the atoms and they are stable chemical elements. In this experiment, the researchers do not want atoms that lose their electrons too easily.
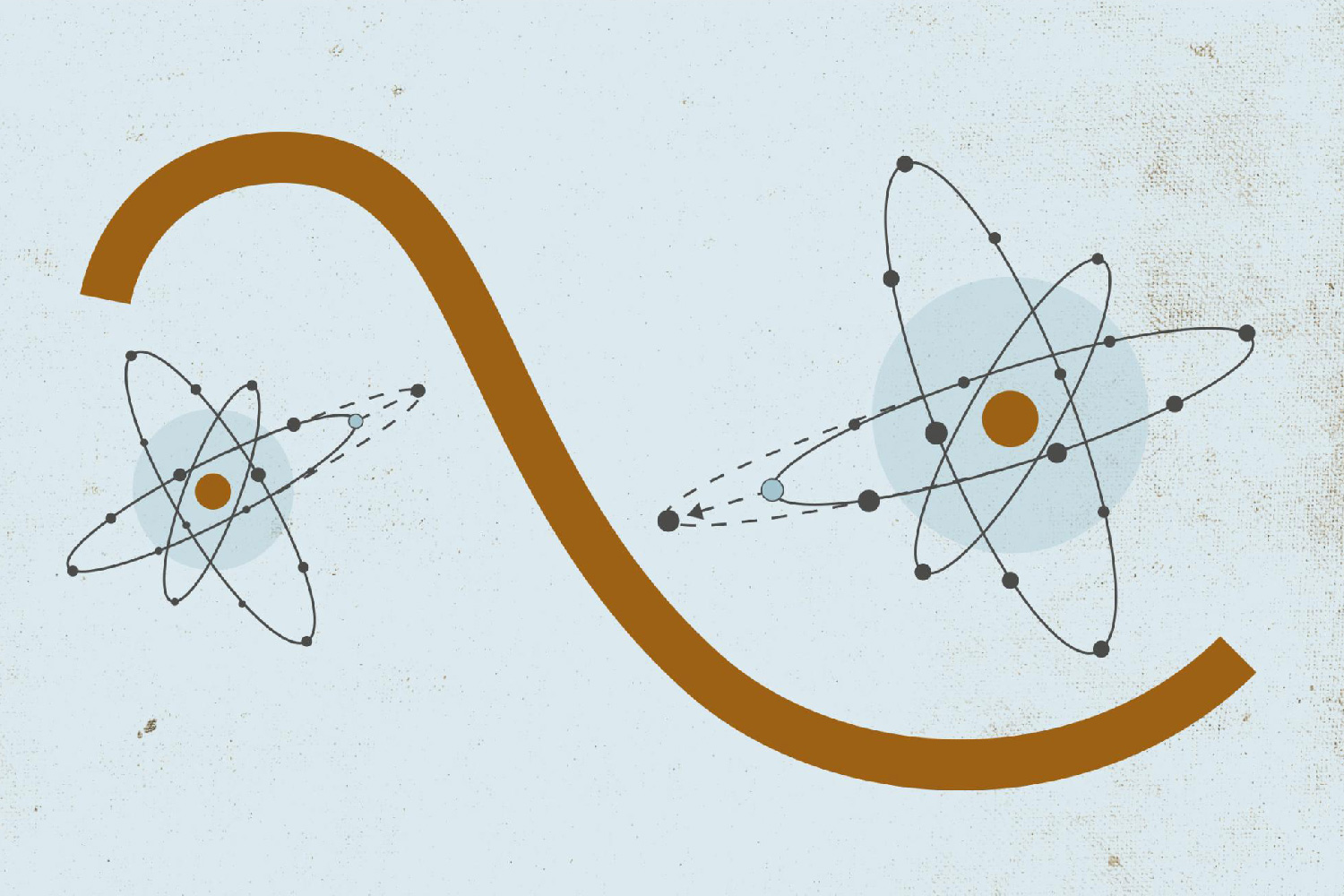
When the laser pulses hit the atom's electron shells, there is a ‘tug of war’ for the electrons between the atomic nucleus and the laser. The laser light is just strong enough to shake the electrons without tearing them away from the nucleus.
This is where the ‘magic’ happens. As the electrons return to their stable orbits around the atomic nucleus, they must release the excess energy absorbed from the pulsed laser. The energy is emitted as flashes of light lasting a billionth of a billionth of a second. To borrow a term from music, this is known as an overtone. The same tone but several octaves higher.
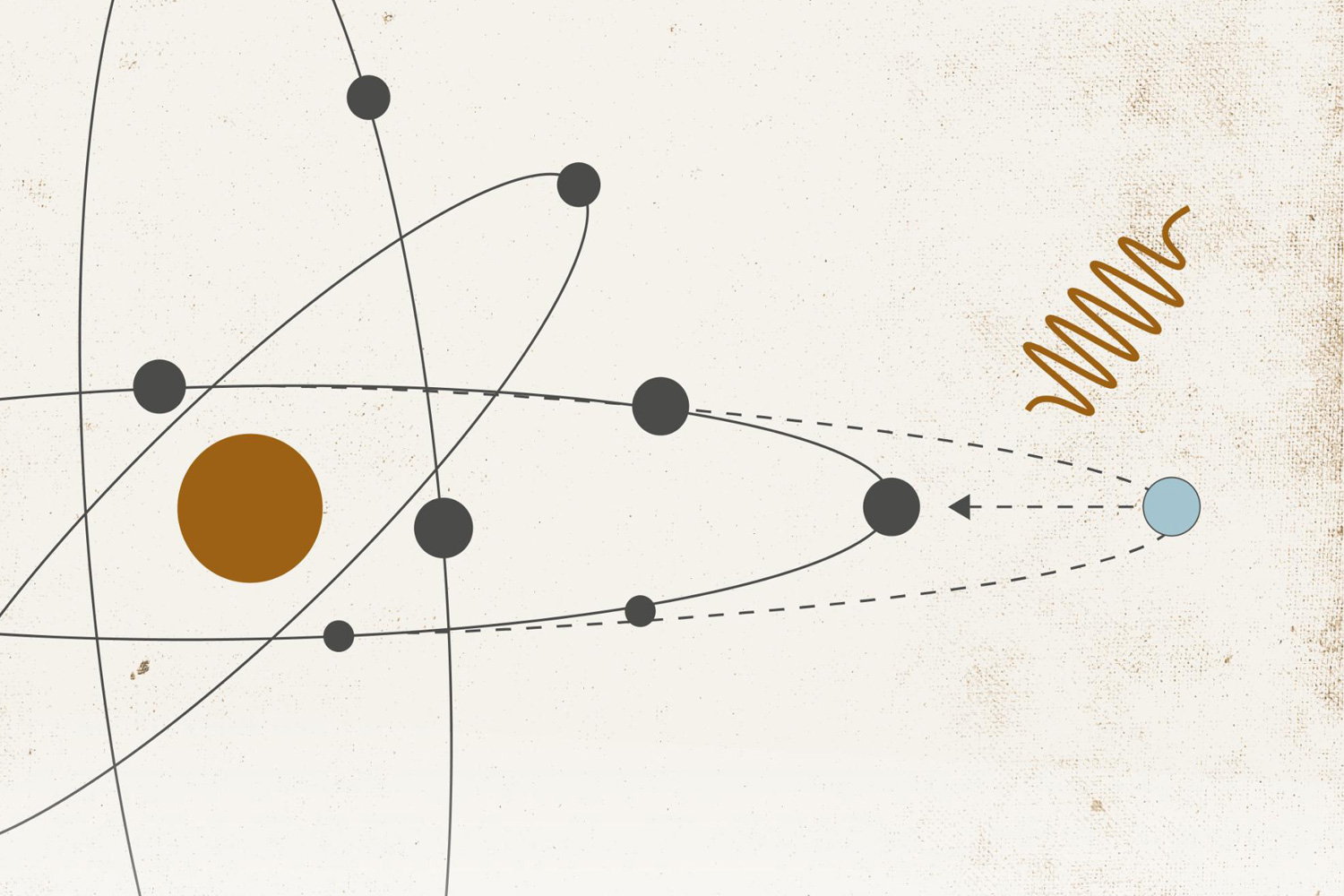

To use the attosecond pulses for measurement purposes, the beam is directed towards a vacuum sealed container containing the object to be studied. The object can be a gas, crystal, metal or other solid material.
How are the measurement done? It depends on what you are trying to find out. Often measurements are taken of light absorption or the number of electrons torn from the nucleus of an atom. This helps us understand the movement of electrons or molecular changes during a chemical reaction. A spectrometer is usually used to register the reading.
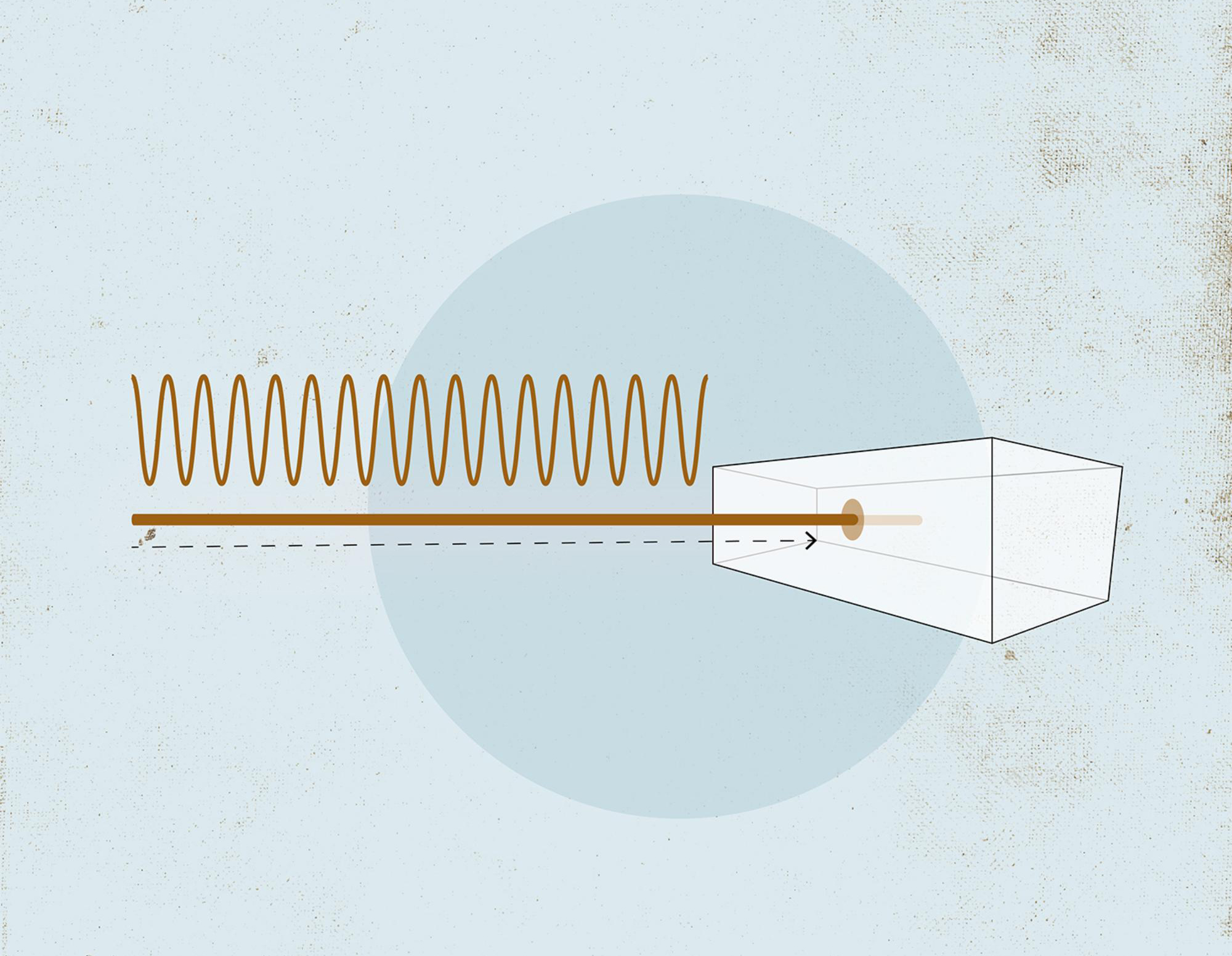

First, the researchers switch on a laser with infrared light pulses. These pulses are on the femtosecond scale, lasting a millionth of a billionth of a second.

A concentrated laser beam is directed at a noble gas. Why a noble gas? Gases allow the irradiation of all the atoms and they are stable chemical elements. In this experiment, the researchers do not want atoms that lose their electrons too easily.

When the laser pulses hit the atom's electron shells, there is a ‘tug of war’ for the electrons between the atomic nucleus and the laser. The laser light is just strong enough to shake the electrons without tearing them away from the nucleus.

This is where the ‘magic’ happens. As the electrons return to their stable orbits around the atomic nucleus, they must release the excess energy absorbed from the pulsed laser. The energy is emitted as flashes of light lasting a billionth of a billionth of a second. To borrow a term from music, this is known as an overtone. The same tone but several octaves higher.

To use the attosecond pulses for measurement purposes, the beam is directed towards a vacuum sealed container containing the object to be studied. The object can be a gas, crystal, metal or other solid material.

How are the measurement done? It depends on what you are trying to find out. Often measurements are taken of light absorption or the number of electrons torn from the nucleus of an atom. This helps us understand the movement of electrons or molecular changes during a chemical reaction. A spectrometer is usually used to register the reading.
The material is also illuminated with another pulse, for example from the infrared laser used previously. By changing the distance between the pulses and repeating the measurement, ‘still images’ are taken that can later be assembled into a film.
